Clinical study results intended to be used in regulatory submissions for gynecologic indications
New study begins on the heels of FDA marketing authorization for use in adults undergoing colectomy procedures
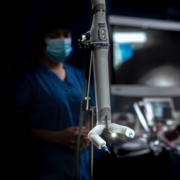
LINCOLN, Nebraska – July 30, 2024 – Virtual Incision Corporation, the developer of the MIRA Surgical System (MIRA), today announced the successful completion of the first surgery in a clinical study assessing the safety and efficacy of its miniaturized robotic-assisted surgery (miniRAS) device in benign hysterectomy procedures.
More than one million women in the U.S. alone undergo surgery for benign gynecologic conditions annually.1 A minimally invasive approach can shorten the hospital stay, reduce blood loss and decrease complication rates compared to open surgery. Robotic-assisted surgery (RAS) devices, like MIRA, could elevate the surgeon’s vision, precision, and control when operating through a small incision, ultimately enabling the techniques to be performed on a broader range of patients.2 However, about 90 percent of U.S. operating rooms (ORs) are still lacking access to RAS, most often due to complex logistics such as dedicated space, specially trained staff, long turnover times and a substantial cost for the equipment.3.
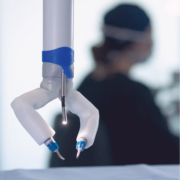
MIRA’s innovative tray-to-table design could offer healthcare facilities the advantages of RAS without requiring them to organize the OR around the device. The miniRAS device’s compact, approximately two-pound (less than one kg), framework is portable and designed to make any OR robot-ready within minutes.
The MIRA Surgical System Hysterectomy Study is an international prospective clinical study designed to evaluate device performance and safety of the MIRA Surgical System when used to robotically assist in benign hysterectomy procedures. Outcomes from this study are planned to support future U.S. and international regulatory submissions for benign gynecologic indications. This study is the second clinical study of MIRA. The first study, under a U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) protocol, supported the De Novo marketing authorization for MIRA in colectomy procedures that was announced in February 2024.
This first surgery was successfully completed at the Geneva University Hospitals by Jean Dubuisson, M.D., Ph.D., a gynecologic surgeon and the principal investigator for the MIRA Surgical System Hysterectomy Study.
“Completing the first hysterectomy with the MIRA Surgical System is an exciting milestone for our hospital and for the entire field of gynecology,” said Dr. Dubuisson. “Robotic-assisted surgery is a valuable tool, but technology access is still a challenge due to cost, time and operating room space. Miniaturization has the potential to overcome these issues and enable more women to have a minimally invasive option for their surgery. We are pleased with the results so far and look forward to further assessing the device in the MIRA Hysterectomy Study.”
“At Virtual Incision, we are anchored in clinical excellence as our first priority,” said Piet Hinoul M.D., Ph.D., chief medical officer of Virtual Incision. “We are encouraged by the results of our clinical and preclinical work to date, and we believe that miniaturized robotic-assisted surgery will have broad applications across a wide variety of procedures. We are grateful to the Geneva University Hospital team for their contributions, as well as to the patients who are willing to participate in this important work. We are excited to reach this milestone as part of our mission to make every operating room robot-ready.
In parallel to conducting the MIRA Surgical System Hysterectomy Study, Virtual Incision is continuing to innovate in the field of miniaturized surgical robotics. New iterations are in design that will enable MIRA to be applied across specialties including general surgery, urology, and other soft tissue and solid organ surgery.
About the MIRA Surgical System
MIRA is the world’s first miniaturized robotic-assisted surgery (RAS) system. Its small, sleek form factor is designed to offer the benefits of RAS during colectomy procedures without the logistical inefficiencies of traditional mainframe robotics. The easily accessible device weighs approximately two pounds (less than one kg) and offers internal triangulation with shoulders, arms, and infinite wrist roll inside of the body. It can be used in any operating room – a dedicated mainframe room is unnecessary. With its drape- and dock-free design and portability, MIRA is quick to set up, clean up, and move between cases. Its conveniently accessible design positions it to be used as a standalone system or a complementary tool for facilities that already own a mainframe. With MIRA, every operating room is RAS-ready.
About Virtual Incision
Virtual Incision is on a mission to simplify robotic-assisted surgery (RAS), so more patients and their surgeons can access its benefits every day. Headquartered in Lincoln, Nebraska, and holding over two hundred patents and patent applications, the company developed MIRA, the first-of-its-kind miniature RAS system. Virtual Incision’s goal is to make every operating room RAS-ready. For more information, visit our website or follow us on LinkedIn and X.
Important Safety Information
The MIRA Surgical System is intended for prescription use only. Patients should talk to their doctor to decide if surgery with a MIRA Surgical System is right for them. For important safety information, indications for use, risks, and warnings, please refer to www.virtualincision.com/safety-information.
Cautionary Note Regarding Forward-Looking Statements
This communication contains statements that constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include but are not limited to, statements regarding our plans, beliefs, expectations, assumptions, and other statements that are not necessarily historical facts. You are cautioned that these forward-looking statements are only predictions and involve risks and uncertainties. Further, any forward-looking statement speaks only as of the date on which it is made, and we do not intend to update or revise any forward-looking statements. This communication also contains market data related to our business and industry which includes projections that are based on several assumptions we believe are reasonable and most significant to the projections as of the date of this communication. If any of our assumptions prove to be incorrect, our actual results may significantly differ from our projections based on these assumptions.
References:
- Life Science Intelligence 2021 Report on Obstetrics and Gynecology.
- Lenfant L, Canlorbe G, Belghiti J, Kreaden US, Hebert AE, Nikpayam M, Uzan C, Azaïs H. Robotic-assisted benign hysterectomy compared with laparoscopic, vaginal, and open surgery: a systematic review and meta-analysis. J Robot Surg. 2023 Dec;17(6):2647–2662. doi: 10.1007/s11701-023–01724‑6. Epub 2023 Oct 19. PMID: 37856058; PMCID: PMC10678826.
- Hazan, A., Perse, J., Coover, P. June 2022. State of the Robotics Surgery Market. Goldman Sachs.
Media Contact:
Jenna Kane
Health+Commerce
jennakane@healthandcommerce.com
(480) 388‑9587
# # #






 Lincoln, Neb. – May 18, 2023 –
Lincoln, Neb. – May 18, 2023 – 