Seasoned medtech leader and former Intuitive Surgical executive to accelerate development and commercialization of the company's next-generation MIRA Surgical System platform and technologies
LINCOLN, Nebraska – June 18, 2025 – Virtual Incision Corporation, the developer of the MIRA Surgical System authorized by the FDA through the De Novo regulatory pathway, today announced the appointment of Jim Alecxih as chief executive officer. Alecxih, a recognized commercial leader in the medical device industry, brings more than 30 years of experience advancing transformative surgical technologies worldwide, including a distinguished tenure at Intuitive Surgical, where he played a pivotal role in the adoption and growth of the da Vinci Surgical System.
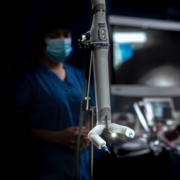
Virtual Incision is now preparing for the accelerated development and commercialization of their next system, M2, with plans for a series of FDA submissions in various specialties. M2 is a miniaturized, single port surgical robotic system designed to be smart, simple and small, with the potential to minimize the cost and complexity of current mainframe cart-based surgical robotic systems. M2 will be optimized to bring robotic-assisted surgery to hospitals and surgical settings that lack access to surgical robotics technologies.
Mr. Alecxih previously served as CEO of DH Medical, an AI software company, and has held executive leadership roles across multiple healthcare startups and growth-stage companies. At Intuitive Surgical, he led U.S. sales and was instrumental in driving adoption of robotic-assisted surgery among hospitals and health systems nationwide.
“The opportunity to lead Virtual Incision at this pivotal time is an extraordinary honor,” said Jim Alecxih, CEO of Virtual Incision. “I believe deeply in the benefits of robotic-assisted surgery to both patients, surgeons and hospitals. M2 has the potential to dramatically expand access to robotic-assisted surgery in rural hospitals, HOPD and ASC settings, and around the world. Virtual Incision’s visionary approach is to ensure that robotic-assisted surgery is more accessible, flexible and scalable for a broader range of operating environments. I’m thrilled to join this talented team and help accelerate our impact on patients and providers.”
“We are delighted to welcome Mr. Alecxih to Virtual Incision at this significant stage in the company’s growth,” said Tyler Stowater, partner at Bluestem Capital and Virtual Incision board member. “Mr. Alecxih’s exceptional leadership skills and deep industry knowledge make him the ideal person to lead us into our next phase of growth and innovation.”
Mr. Alecxih joins Virtual Incision at a time of significant momentum. In 2025, the company has been honored as a finalist or winner in three prestigious innovation awards:
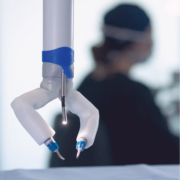
Virtual Incision’s MIRA Surgical System is designed to offer a portable, scalable solution for minimally invasive procedures with a small footprint and a simple setup. With M2, the company aims to expand clinical capabilities and will continue redefining the future of robotic-assisted surgery.
About the MIRA Surgical System
MIRA is the world’s first miniaturized robotic-assisted surgery (RAS) system. Its small, sleek form factor is designed to offer the benefits of RAS during colectomy procedures without the logistical inefficiencies of traditional mainframe robotics. The easily accessible device weighs approximately two pounds (less than one kg) and offers internal triangulation with shoulders, arms, and infinite wrist roll inside of the body. It can be used in any operating room – a dedicated operating room is unnecessary. With its drape- and dock-free design and portability, MIRA is quick to set up, clean up, and move between cases. Its conveniently accessible design positions it to be used as a standalone system or a complementary tool for facilities that already own a legacy surgical robotic system. With MIRA, every operating room is RAS-ready.
About Virtual Incision
Virtual Incision is on a mission to simplify robotic-assisted surgery (RAS), so more patients and their surgeons can access its benefits every day. Headquartered in Lincoln, Nebraska, and holding over two hundred patents and patent applications, the company developed MIRA, the first-of-its-kind miniature RAS system. Virtual Incision’s goal is to make every operating room RAS-ready. For more information, visit our website or follow us on LinkedIn.
Important Safety Information
The MIRA Surgical System is intended for prescription use only. Patients should talk to their doctor to decide if surgery with a MIRA Surgical System is right for them. For important safety information, indications for use, risks, and warnings, please refer to www.virtualincision.com/safety-information.
Cautionary Note Regarding Forward-Looking Statements
This communication contains statements that constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include but are not limited to, statements regarding our plans, beliefs, expectations, assumptions, and other statements that are not necessarily historical facts. You are cautioned that these forward-looking statements are only predictions and involve risks and uncertainties. Further, any forward-looking statement speaks only as of the date on which it is made, and we do not intend to update or revise any forward-looking statements. This communication also contains market data related to our business and industry which includes projections that are based on several assumptions we believe are reasonable and most significant to the projections as of the date of this communication. If any of our assumptions prove to be incorrect, our actual results may significantly differ from our projections based on these assumptions.
Media Contact:
Ashlynn Meyer
Virtual Incision
ashlynn.meyer@virtualincision.com
###